The Technology
Innovation with proven results
Innovation with proven results
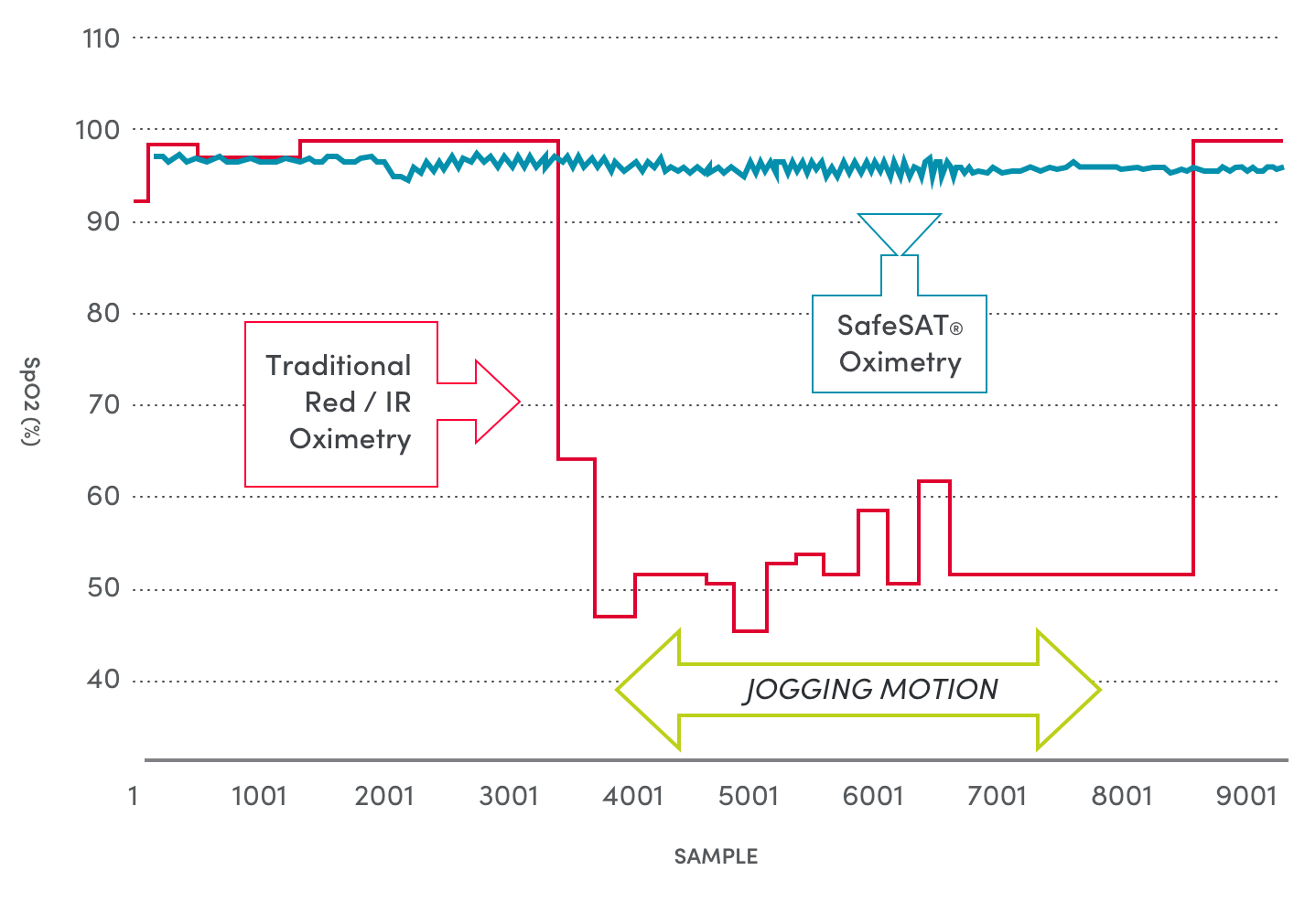
Healthcare providers need the highest possible accuracy to monitor patients’ key vital signs. But the current oximeter devices are only accurate when patients are motionless. And variations in skin coloration, tattoos and nail polish pose further challenges for traditional pulse oximeters.

Medtor’s FDA cleared SafeSAT® technology provides a breakthrough in vital signs monitoring. The patented spectral sensor technology delivers the most accurate heart rate and blood oxygen readings available, filtering out movement and other interfering factors, such as skin tone.

Medtor’s technology produces two separate and independent data streams that provide enhanced precision and accuracy. One data stream is based on two-wavelength illumination, and the other reveals precise tissue properties from spectral white light absorption—an innovation in precision and accuracy.
Medtor’s two data streams are processed by a proprietary algorithm to derive stable composite data for blood oxygenation and heart rate.
The small hardware footprint of the SafeSAT® sensor can be packaged in a variety of ways, giving hardware makers the flexibility needed to make products as compact as possible with no sacrifice in accuracy.
The SafeSAT® technology provides stable and accurate Heart Rate data, even on highly active subjects and under conditions where current optical sensors are unable to provide meaningful or accurate information.

SafeSAT® sensor power consumption is minimal by design, which allows sensor packaging into fitness trackers, smart watches, and other mobile electronics. The sensor can monitor vital signs throughout the day with minimal battery drain.
Our SafeSAT® technology is protected by several issued and pending patents, granted worldwide. We continue to expand the features of our technology platform and expect further innovations protect in new patent filings.
US Patent 8 271 063
US Patent 6 889 153
US Patent 7 124 048
US Patent 10 485 463
US Patent 11 350 861
EP 1 307 708
Canada 2419054
Austria AT0000366913
Australia AU0200181231
Germany DE 60129332
PCTWO 2008002405
PCT US14/060004
Medtor’s FDA cleared SafeSAT technology has been proven to significantly outperform current technology in a series of independent clinical studies, delivering the highest motion-tolerant Sp02 accuracy ever available. Our technology moves vital sign monitoring from an old technology through a doorway to tomorrow.
The small hardware footprint of the SafeSAT® sensor can be packaged in a variety of ways, giving hardware makers the flexibility needed to make products as compact as possible with no sacrifice in accuracy.
Performed November 2014
7 Subjects
Skin pigmentation light to dark
2 SafeSAT® Oximeters with finger clip sensors, one on each hand were able to acquire both red/ir and white SpO2 data for all subjects for the full saturation range
Both red/ir and white-based SpO2 data follow well defined transfer functions with respect to the reference oximeter used by Clinimark with ARMS value of 2.56 for the entire saturation range from 99% to 70%
Both SafeSAT® systems were able to acquire accurate data on all study subjects, independent of skin type
Study validates the ability of the SafeSAT® oximeter technology to provide a parallel white LED based SpO2 data stream leveraging the proprietary spectral sensor technology
Performed March 2015
15 Subjects
Skin pigmentation light to dark
The wrist sensor delivered white LED derived values with ARMS of 1.9% SpO2 over the full saturation range
The finger clip sensor connected to the SafeSAT® oximeter white-LED derived saturation data delivered values with an ARMS of 3.2% SpO2 between 70% and 100% saturation compared to the Covidien oximeter
Our SafeSAT® sensors (both reflectance and transmissive) and proprietary algorithm have been evaluated in several independent clinical studies referenced both to transfer standard oximeters and blood gas based reference data. Our sensors and hardware were able to produce results that reduced absolute errors more than three-fold and root mean standard deviations more than two-fold when referenced to current state-of-the-art clinical sensors and oximeters.
Performed May 2015 Clinimark Hypoxia Lab
10 Subjects
Skin pigmentation light to dark
Greatly enhanced real-time stability of SpO2 and read-through motion ability of SafeSAT® finger and wrist reflective sensors
Compared to Covidien Max adhesive finger sensors, SafeSAT® non-adhesive finger sensor performs with 3x greater motion tolerance
Compared to adhesive MaxFact Forehead sensor, the SafeSAT® non-adhesive wrist sensor still provides a 30% reduction in STDEV of oxygen saturation data
The SafeSAT® Oximeter effectively filters out motion artifacts in both finger and wrist site when compared to Covidien adhesive finger and forehead sensors placed on the same subject
Performed Feb 2016 Clinimark Hypoxia Lab
12 Subjects
Skin pigmentation light to dark
Total motion events out of spec (>4 points absolute error for SpO2 or HR)
Results
Medtor SafeSAT®: 43.0% for SpO2 and 45.1% for HR
Masimo Racical: 44.4% for SpO2 and 49.1% for HR
Covidien N600x: 65.7% for SpO2 and 58.5% for HR
35% reduction in out of spec SpO2 events for Medtor vs. Covidien
Performed December 2016 Toulon France
Study compared mean SpO2 bias, SpO2 standard deviation and relative data availability referenced to SaO2 blood gas reference on 21 patients admitted to the site ICU
15 Male & 6 Female Subjects
Age Range : 21 to 87 years
5 patients with Septic Shock
14 patients considered 'challenging' by PI
Direct comparison of Medtor SafeSAT™ oximeter to Masimo Radical 7 and Nihon Koden
30 min recorded data for 21 patients and Co-ox blood-gas reference sample taken mid-point
'Real-world' study environment with majority very challenging patients recorded
Results
Medtor performed best in Mean Bias & Standard-Deviation vs. Blood-gas sample
Medtor performed best in Data Availability on the "Challenging patient' group
Performed December 2017 UCSF Hypoxia Lab
12 Subjects
Skin pigmentation light to dark
Finger Clip sensors for all systems compared
Two types of random subject induced motion during hypoxia
Data collected at an independent clinical study laboratory in the United States
Results (FDA Guidance requires Arms < 3.0)
SafeSAT™ Oximeter Arms 70 to 100% SpO2 - 2.69
Nellcor Oximax Arms 70 to 100% SpO2 - 3.43
Masimo Rainbow: Arms 70 to 100% SpO2 - 5.64
Please reach out to learn more about developing our technology.